-
1
-
2
-
3
-
4
-
5
-
6
-
7
-
8
-
9
-
10
-
11
-
12
-
13
-
14
-
15
-
16
-
17
-
18
-
19
-
20
-
21
-
22
-
23
-
24
-
25
-
26
-
27
-
28
-
29
-
30
-
31
-
32
-
33
-
34
-
35
-
36
-
37
-
38
-
39
-
40
-
41
-
42
-
43


본문내용
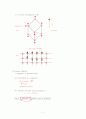
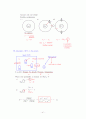
N개 independent particles, or electron (ideal gas)
N개 molecules confined in a box of volume
What is the pressure ?
sol1) using basic physics
→ we get
→ “Eq. of state for ideal gas"
이상기체 상태 방정식
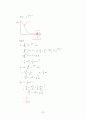
Quantum mechanical approach
focusing on one particle
Schrodinger eq.
…… ①
set U = 0
→ What is the solution ?
Eigenfunction satisfy the boundary condition At boundary
solution)
②
Here, integers
Inserting ② into the Schrodinger eq.
We have "eigenvalue E"
③
Where,
cf: A simple harmonic oscillator
Quantumized
양자화
ⅰ) the lowest energy →
ⅱ) the next excited energy
energy
pressure
free energy one particle
→ Pressure by a particle
N개 particle
in agreement with classical "eq. of the state"
N개 particle
- Diffusive contact
- Chemical potential
Thermal contact
, vary but , = fixed
thermal equilibrium
Diffusive contact
allow particle transfer
can vary
diffusive!!
althought vary
What is the minimum physical quantity?
①
using
②
eq② → eq①
We have
Condition for diffusive equilibrium
New thermal physics quantity define.
“Chemical potential"
→ Note) almost same as "fermi energy" in solid-state physics
In general
Ex) N개 particle are confined into a box of volime V.
What is the chemical potential of the system?
sol)
is one particle partition function
Here
What is
where
“Quantum concentration" (양자 밀도)
Review
diffusive contact
diffusive equilibrium
chemical potential
quantum concentration
diffusive equilibrium
More extended condition
Ex) N개 ideal gas
where
Quantum concentration & chemical potential
Apply to and gas
only one개
Compare
electron gas & gas
by 4,000 times
by 60 times
by 24,000 times
for same density n.
Ex)
ⅰ) 300K, air
so dilufed (희석)
“classical"
ⅱ) inside metal 금속
n approach to “Quantum mechanically"
Ex) Atmospheric pressure change with altitude (Height 고도)
modeling Air
Assume
diffusive ithothermic(등온)
contact Chemical potential
diffusive equilibrium
①
ideal gas eq. of state
②
②→①
“barometric pressure eq"
Airplane altitude h=8km
apply , T=300K
Chemical potential
Pressure
In general
①
differenciate ① with respect for N:
use ,
Alternative expression of
※ Start from entropy
The Extended thermal dynamic 1st law
In general
“extended 1st law"
Grand canonical distribution (=Gibbs distribution)
reservoir
Heat & Particle reservoir
system
What is the probability for the system to be in a specific state and particle number
⇒ “Gibbs probability or Gibbs distribution"
Math: Taylor expansion
∴We have
Gibbs factor Boltzmann factor
Absolute prob.
Application of Gibbs factor
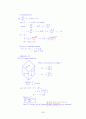
1) inpurity in semiconductor
2) O2 Adsorption in Hemoglobin
sol) 1) Inpurity in Si
Assume: only one-orbital
Possible configuration
ⅰ) ⅱ) ⅲ)
No electron
infinite
distance
Ex)
binding energy
Adsorption (흡착) on the protein
blood (혈액)
"Hemoglobin"
Oxygen reservoir
red color in blood
In a given Oxygen density, Pressure, Temperature
What is the probability, or fraction for ??
ⅰ) N=0
ⅱ)
①
What is ?
in terms of
Approximation: treat → ideal gas
②
② → ①
ideal gas
③
∴ in terms of pressure
Plot
N개 molecules confined in a box of volume
What is the pressure ?
sol1) using basic physics
→ we get
→ “Eq. of state for ideal gas"
이상기체 상태 방정식
Quantum mechanical approach
focusing on one particle
Schrodinger eq.
…… ①
set U = 0
→ What is the solution ?
Eigenfunction satisfy the boundary condition At boundary
solution)
②
Here, integers
Inserting ② into the Schrodinger eq.
We have "eigenvalue E"
③
Where,
cf: A simple harmonic oscillator
Quantumized
양자화
ⅰ) the lowest energy →
ⅱ) the next excited energy
energy
pressure
free energy one particle
→ Pressure by a particle
N개 particle
in agreement with classical "eq. of the state"
N개 particle
- Diffusive contact
- Chemical potential
Thermal contact
, vary but , = fixed
thermal equilibrium
Diffusive contact
allow particle transfer
can vary
diffusive!!
althought vary
What is the minimum physical quantity?
①
using
②
eq② → eq①
We have
Condition for diffusive equilibrium
New thermal physics quantity define.
“Chemical potential"
→ Note) almost same as "fermi energy" in solid-state physics
In general
Ex) N개 particle are confined into a box of volime V.
What is the chemical potential of the system?
sol)
is one particle partition function
Here
What is
where
“Quantum concentration" (양자 밀도)
Review
diffusive contact
diffusive equilibrium
chemical potential
quantum concentration
diffusive equilibrium
More extended condition
Ex) N개 ideal gas
where
Quantum concentration & chemical potential
Apply to and gas
only one개
Compare
electron gas & gas
by 4,000 times
by 60 times
by 24,000 times
for same density n.
Ex)
ⅰ) 300K, air
so dilufed (희석)
“classical"
ⅱ) inside metal 금속
n approach to “Quantum mechanically"
Ex) Atmospheric pressure change with altitude (Height 고도)
modeling Air
Assume
diffusive ithothermic(등온)
contact Chemical potential
diffusive equilibrium
①
ideal gas eq. of state
②
②→①
“barometric pressure eq"
Airplane altitude h=8km
apply , T=300K
Chemical potential
Pressure
In general
①
differenciate ① with respect for N:
use ,
Alternative expression of
※ Start from entropy
The Extended thermal dynamic 1st law
In general
“extended 1st law"
Grand canonical distribution (=Gibbs distribution)
reservoir
Heat & Particle reservoir
system
What is the probability for the system to be in a specific state and particle number
⇒ “Gibbs probability or Gibbs distribution"
Math: Taylor expansion
∴We have
Gibbs factor Boltzmann factor
Absolute prob.
Application of Gibbs factor
1) inpurity in semiconductor
2) O2 Adsorption in Hemoglobin
sol) 1) Inpurity in Si
Assume: only one-orbital
Possible configuration
ⅰ) ⅱ) ⅲ)
No electron
infinite
distance
Ex)
binding energy
Adsorption (흡착) on the protein
blood (혈액)
"Hemoglobin"
Oxygen reservoir
red color in blood
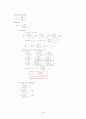
In a given Oxygen density, Pressure, Temperature
What is the probability, or fraction for ??
ⅰ) N=0
ⅱ)
①
What is ?
in terms of
Approximation: treat → ideal gas
②
② → ①
ideal gas
③
∴ in terms of pressure
Plot
추천자료
 고대의 자연철학과 과학과 기술
고대의 자연철학과 과학과 기술 나는 물리학을 가지고 놀았다 노벨상 수상자 리처드 파인만의 삶과 과학
나는 물리학을 가지고 놀았다 노벨상 수상자 리처드 파인만의 삶과 과학 [과학교과평가]과학과평가(과학교육평가)의 분류와 과학과평가(과학교육평가)의 측정, 과학과...
[과학교과평가]과학과평가(과학교육평가)의 분류와 과학과평가(과학교육평가)의 측정, 과학과... [과학과교육평가]과학교육평가(과학과평가)의 원칙, 과학교육평가(과학과평가)의 목적과 기능...
[과학과교육평가]과학교육평가(과학과평가)의 원칙, 과학교육평가(과학과평가)의 목적과 기능... 과학과수업(교육, 학습) 성격과 목적, 과학과수업(교육, 학습) 탐구이론과 학습교재, 과학과...
과학과수업(교육, 학습) 성격과 목적, 과학과수업(교육, 학습) 탐구이론과 학습교재, 과학과... 과학과수업(교육) STS(과학기술사회)학습, 과학과수업(교육) 현장학습, 과학과수업(교육) 발...
과학과수업(교육) STS(과학기술사회)학습, 과학과수업(교육) 현장학습, 과학과수업(교육) 발... 과학과학습(수업, 교육) 목적과 구성주의적 관점, 과학과학습(수업, 교육) 도서관 활용과 개...
과학과학습(수업, 교육) 목적과 구성주의적 관점, 과학과학습(수업, 교육) 도서관 활용과 개... 과학과교육(과학학습)의 목적, 과학과교육(과학학습)의 지역적 맥락, 과학과교육(과학학습)의...
과학과교육(과학학습)의 목적, 과학과교육(과학학습)의 지역적 맥락, 과학과교육(과학학습)의... 동양철학에 있어서 자연주의와 과학기술주의
동양철학에 있어서 자연주의와 과학기술주의 [과학교육]과학과교육(학습)의 성격, 과학과교육(학습)의 목표, 과학과교육(학습)의 과정, 과...
[과학교육]과학과교육(학습)의 성격, 과학과교육(학습)의 목표, 과학과교육(학습)의 과정, 과... 과학과(과학교육, 과학수업) 성격, 과학과(과학교육, 과학수업) 목표, 과학과(과학교육, 과학...
과학과(과학교육, 과학수업) 성격, 과학과(과학교육, 과학수업) 목표, 과학과(과학교육, 과학... 유아 아동 과학 물리(고무호수, 고무줄 실험) (만5세) - 건강한 몸과 마음 : 목소리는 어떻게...
유아 아동 과학 물리(고무호수, 고무줄 실험) (만5세) - 건강한 몸과 마음 : 목소리는 어떻게... 유아 과학교육을 위한 환경 - 심리적 환경과 물리적 환경 및 과학 교육 환경의 안전 - 물리적...
유아 과학교육을 위한 환경 - 심리적 환경과 물리적 환경 및 과학 교육 환경의 안전 - 물리적... 누리과정에서 과학교육의 방향, 성격, 목표, 세부내용을 이해한 후 이와 연계할 수 있는 과학...
누리과정에서 과학교육의 방향, 성격, 목표, 세부내용을 이해한 후 이와 연계할 수 있는 과학...